From the DukeHealth.org archives. Content may be out of date.
From the DukeHealth.org archives. Content may be out of date.
New Keratoconus Treatment May Prevent Need for Corneal Transplant
Cross-Linking Procedure Can Halt Corneal Changes, Preserve Vision

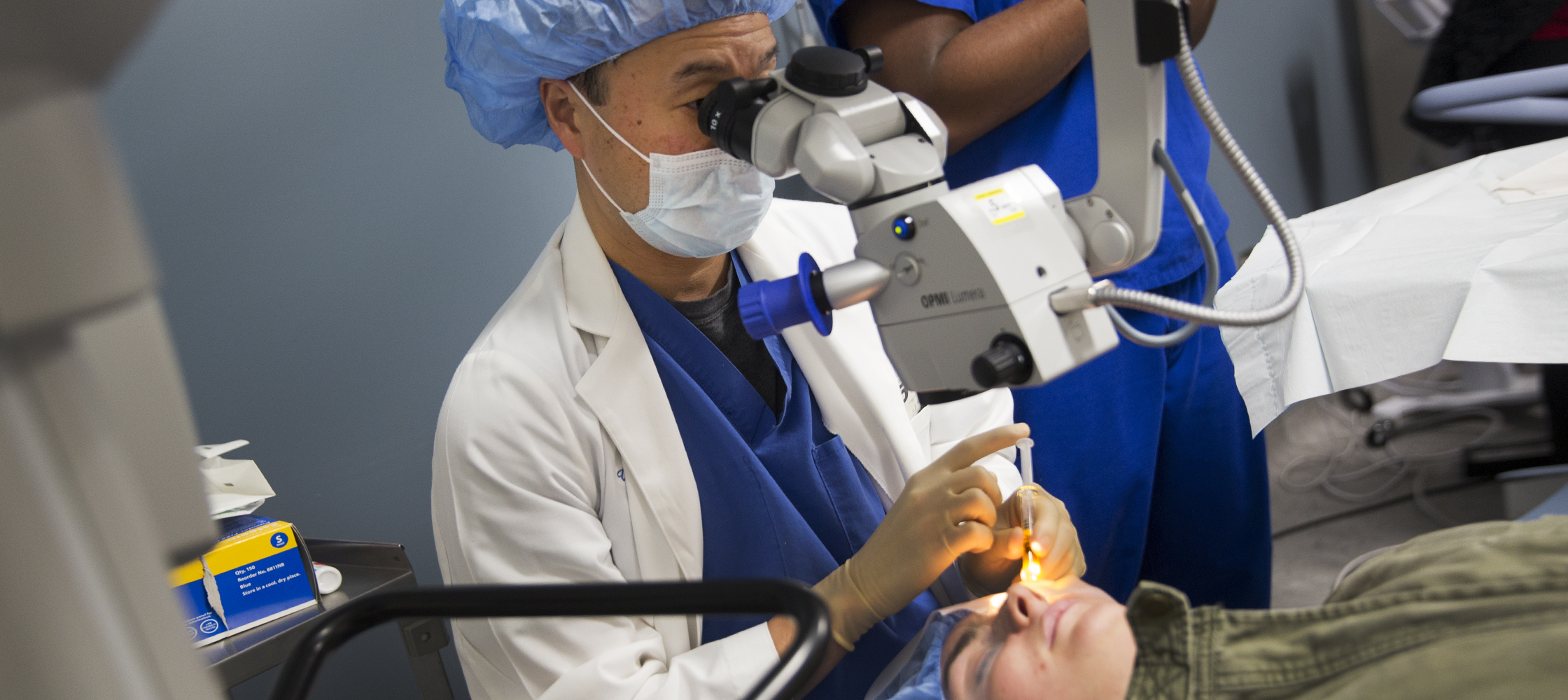
Riboflavin drops are placed in the eye during corneal collagen cross-linking treatment
Corneal collagen cross-linking, an FDA-approved treatment for the eye disease keratoconus, can preserve vision and prevent the need for a corneal transplant.
Cross-Linking Received FDA Approval in 2016
Until recently, people with keratoconus had few treatment options. The best their eye doctors could offer were increasingly stronger eyeglasses, special contact lenses, and eventually, in many cases, a corneal transplant. In spring 2016, the FDA approved a new treatment for this condition, which causes your corneas (the clear outer covering of the eye) to bulge gradually into a cone shape, distorting your vision. That treatment, called collagen cross-linking, or CXL, is now available at Duke Eye Center, the first provider in North Carolina to offer it.
“It’s a long-awaited and very exciting development,” said Duke corneal specialist Terry Kim, MD. “Many patients and surgeons in the U.S. have been awaiting this procedure’s approval, as it has been used successfully in Europe for more than 10 years."
How Does Corneal Cross-Linking Work?
The treatment uses vitamin B2 (riboflavin) plus ultraviolet light to strengthen the collagen, a fibrous protein that supports the structure of your cornea. It’s performed on an outpatient basis and takes about an hour and a half.
The ophthalmologist removes the outer layer of cells (epithelium) from your cornea using a special instrument, then places riboflavin drops in your eye. Next, UV light is shone on the eye to activate the riboflavin and stiffen your cornea. Healing typically takes five to seven days, while you wear a bandage contact lens.
Depending on your circumstances, your eye doctor may also offer intracorneal ring segments, which are plastic crescent-shaped devices that are inserted into the cornea to help support its shape and structure.
“Usually keratoconus occurs in both eyes, so typically we would treat the second eye about a month or two later,” said Dr. Kim.
Cross-Linking Stops Keratoconus from Progressing
Some patients may notice a bit of improvement in their vision following the procedure but, said Dr. Kim, “It’s not going to be drastic. The primary goal is to stabilize your corneas, to keep them from getting worse.” That can help you avoid a corneal transplant, which is major surgery that carries significant risks, including rejection of the donor tissue.
Researchers are looking into whether cross-linking may be combined with vision correction surgery in the near future.
Who Is a Candidate for Cross-Linking?
Because the cornea stiffens naturally with age, the progress of keratoconus tends to slow as you get older. For this reason, Dr. Kim said, “Our ideal candidates are in the younger age spectrum -- up through their 40s.” He noted that severe allergy sufferers, who can develop keratoconus from frequent eye rubbing, are at risk into their older ages and may also benefit from the treatment. The FDA has approved the procedure for people ages 14 to 65.
You may not be able to have cross-linking treatment if your cornea is too scarred or too thin, or if you have an active infection or a problem with the surface of your eye, such as severe dry eye. Also, it’s important to note that most insurance companies don’t currently cover the procedure.