 From the DukeHealth.org archives. Content may be out of date.
From the DukeHealth.org archives. Content may be out of date.
The COVID-19 Vaccine Is Here: Continue to Practice Safety Precautions
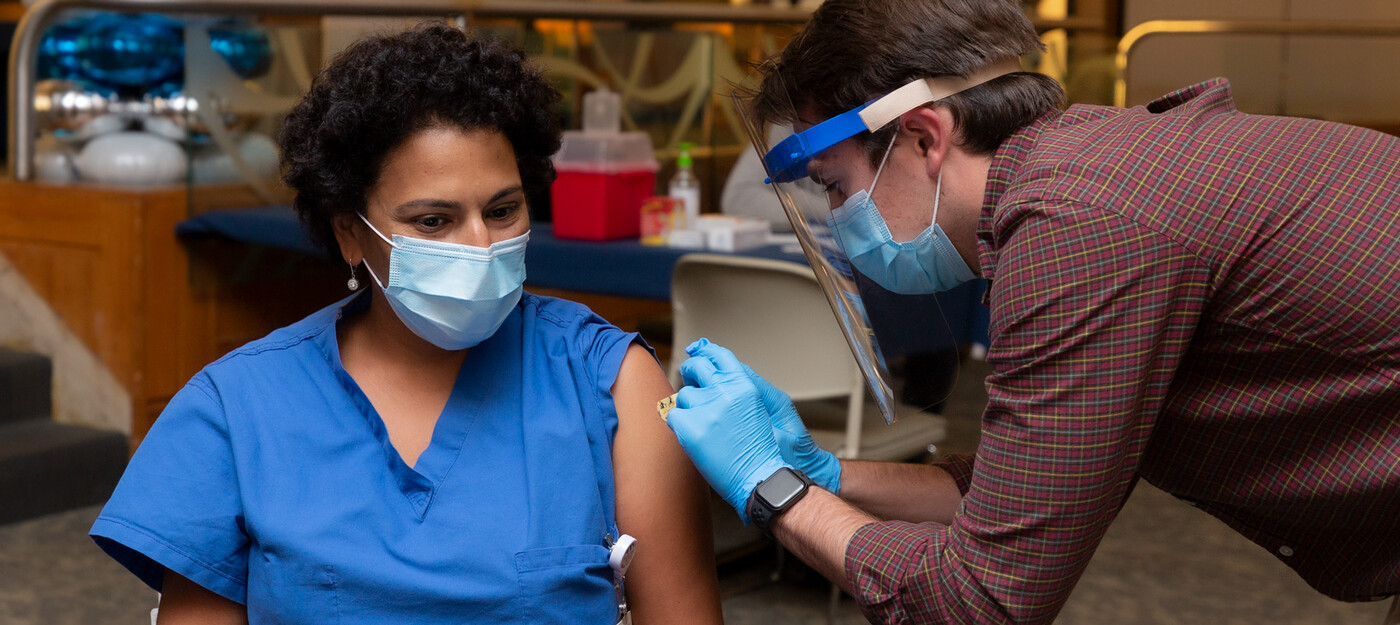
Duke Health has started vaccinating health care workers at high risk for COVID-19 and plans to begin vaccinating patients at high risk in the early months of 2021. Despite the good news, they stress that everyone continue to follow safety precautions -- wear a mask, maintain physical distance, practice proper hand washing, and clean high-touch surfaces -- as we head into the busy holidays and the new year.
The Vaccine Is a Game Changer
“The vaccine is a real game changer, and the safety data are outstanding,” said Dr. Cameron Wolfe, MBBS, a Duke infectious disease expert. The number of people who participated in the clinical trials is “more than we normally see in phase III trials.” However, because Duke doesn’t know how many vaccine doses it will receive or when, it’s not possible to predict how many people can be vaccinated quickly.
“As we get the vaccine into communities, we have to stay focused on staying safe and healthy over the coming weeks and months.” Dr. Wolfe said.
Data Show the COVID-19 Vaccine Is Safe and Effective
Two vaccines are approved by the FDA for emergency use: one is produced by Pfizer; the other by Moderna Therapeutics. Dr. Wolfe and Geeta Swamy, MD, a Duke maternal-fetal medicine specialist and vaccine expert, say the data show both vaccines are equally effective and safe for most adults.
There are some caveats. For example, the effectiveness of the vaccine may be reduced in people with cancer, with an autoimmune disease, or who are immune-suppressed due to another condition. Dr. Wolfe said people who experience severe allergic responses (such as people who carry an EpiPen®) or who have had severe reactions to vaccines, should talk to their doctor to see if the risks of the vaccine outweigh the benefits.
Right now, the vaccines are not approved for use in children because they were not part of the initial clinical trials. That may change, as clinical trials in children are underway.
Talk to Your Doctor If You Are Pregnant or Breastfeeding
The trials also did not include pregnant women who are at higher risk for complications related to COVID-19. “Theoretically, there is no reason to think the vaccine would cause them harm or harm the fetus based on the studies," said Dr. Swamy. The same is true for breastfeeding mothers who also were not part of the vaccine trials. “The likelihood of any concern for the baby is incredibly low,” Dr. Swamy said. “We know the likelihood of transmitting infection is from parents. Protecting ourselves is going to be our best bet.”
If you are pregnant or breastfeeding, Dr. Swamy said it’s important you talk to your doctor about the risks and benefits so you can determine if getting the vaccine is right for you.
When and How People Will Get the Vaccine
Duke Health doesn't know “how many vaccines we will get over the coming weeks,” said Dr. Tom Owens, MD, president of Duke University Hospital. Because the Pfizer vaccine requires special cold storage, it will be more difficult for private practices to house and distribute it. As the situation evolves, “we’ll learn more about how to work with community providers,” he said. That may include chain drug stores like CVS and Walgreens, as well as vaccine centers that may be set up by Duke and UNC.
At the same time, the North Carolina Department of Health and Human Services has clear guidelines on who will get the vaccine based on their risk for developing COVID-19.
"During December and January, the first group of people include front line health care workers and people who live and work in long-term care facilities," Dr. Wolfe said. The next group will be people with risk factors such as immunosuppression, heart disease, chronic lung disease, obesity, and diabetes. “We will factor in age and these co-morbidities to tee up people accordingly and reach out to people to schedule their vaccines.”
If you are a Duke patient, that communication will take place through Duke My Chart. If you don’t already have an account, you can use this link to sign up for one.
“There is a light at the end of the tunnel. We’re close. But if we start letting our guard down, it will be much worse.”
After You Get the Vaccine
Once you get vaccinated, the CDC encourages everyone to sign up for V-Safe, a smartphone app that will use text messaging and web surveys to inform the CDC of any side effects that may occur in the seven days following the doses. “This is completely voluntary,” Dr. Swamy said.
An antibody response can be expected within 10 to 14 days after the initial vaccine, Dr. Wolfe said, and will be enhanced after the second dose. You will make an appointment for that second dose when you get your initial vaccine.
The antibody response should last several months but right now, how long is unclear. Additional studies will help determine when or if additional vaccines will be needed.
Because so much is unknown about how long antibodies last, people who have recovered from COVID-19 should also get vaccinated.
Continue to Practice Safety Precautions
Most important, however, is that everyone maintain the safety precautions that are in place while waiting to get vaccinated and after they’ve been vaccinated. “Just because you have the vaccine doesn’t mean you drop the ball,” Dr. Wolfe said.
Wear a mask, maintain physical distance, practice regular hand washing and wash high-touch surfaces, said Michael Datto, MD, PhD, Associate Vice President for Duke University Health System Clinical Laboratories. “If you’re symptomatic, get tested.” North Carolina has testing locations across the state. If you think you’ve been exposed, self-quarantine.
“There is a light at the end of the tunnel, Dr. Datto said. “We’re close. But if we start letting our guard down, it will be much worse.”





